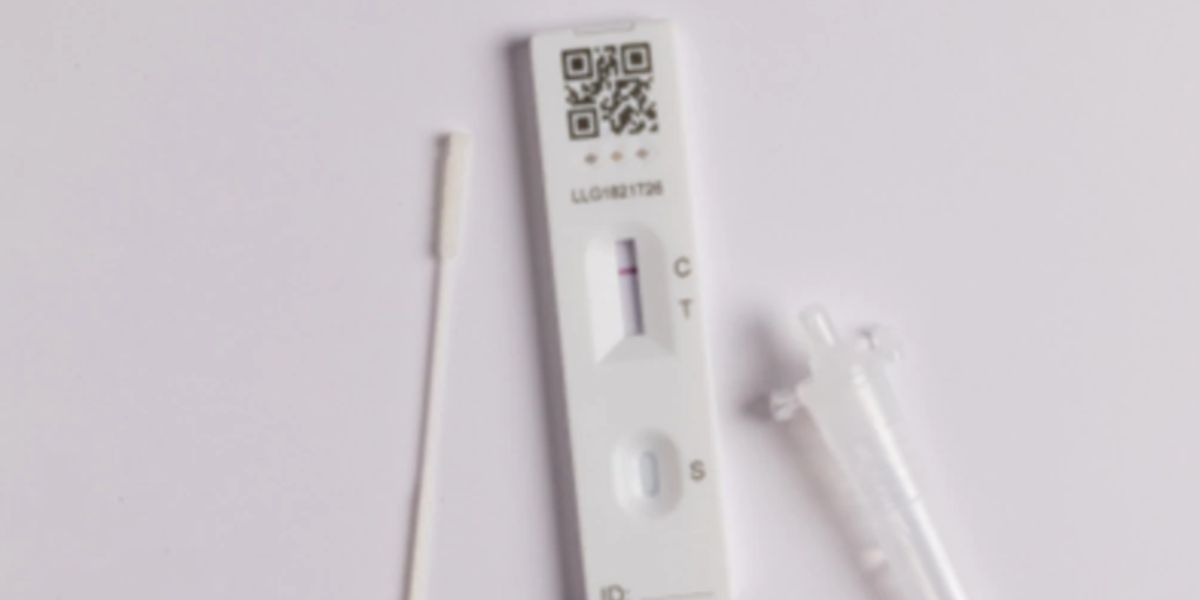
In an Australian-first and huge step in STI prevention for Australian women, the TGA approves Touch Biotechnology’s new sexually transmitted infection (STI) self-test kit for Chlamydia and Gonorrhoea, providing greater privacy and fast results.
The first of its kind in Australia, the highly accurate self-test kit detects Chlamydia and Gonorrhoea with one swab sample, producing a positive or negative result for both infections in 15 minutes.
STIs affect almost one in six (16%) Australians in their lifetime and over the last 10 years, numbers have increased at a concerning rate in Australia, with rates of Chlamydia and Gonorrhoea increasing by 26% and 157% respectively.
Chlamydia is the most reported communicable disease in Australia, with over 110,000 reported cases in 2023, but often appears asymptomatic and as a result, some cases are undiagnosed, with people reluctant to test for the infection due to perceived stigma or lack of awareness.
Gonorrhoea presents itself with pain or discomfort in the throat, eyes, cervix and other areas but similarly, it is often mistaken for other infections and remains undiagnosed.
“For women in particular, STIs such as gonorrhoea and chlamydia are major causes of pelvic inflammatory disease and infertility if they go undiagnosed and untreated,” General Manager at Touch Biotechnology, Mazz Gencer warns.
“These infections can also cause complications to pregnancy and birth because of the risk of mother-to-child transmission. That’s why it’s incredibly important for women to test for STIs when experiencing even the smallest of symptoms or are concerned about a partner’s sexual activity.”
The TGA-approved Chlamydia and Gonorrhoea self-test will be available in the upcoming weeks to purchase at pharmacies, and distributors, and online across the country, to be completed discreetly at home.
Prior to this announcement, the only option for women to test for Gonorrhoea and Chlamydia was to complete an individual lab or PCR test. This invasive process could be costly and takes too long, with results typically taking one to three days.
“The introduction of the first rapid self-test kit for Chlamydia and Gonorrhoea will aid in the prevention of STIs because with increased access to accurate, private testing, we hope it will empower women to take the first step towards receiving a diagnosis.” said CEO of Sydney-based Touch Biotechnology, Matt Salihi.
“It marks significant progress in improving the options for female sexual health in Australia, hopefully reducing the number of infections that go undiagnosed and enabling more people to receive appropriate and timely treatment for infection that prevents further health complications.”
“It’s incredibly important for women, and men, to consider the risks of unprotected sex because despite the perceived stigma, STIs impact so many Australians amounting to much more than uncomfortable symptoms.”
“While this self-test can aid early detection, comprehensive STI screening and follow-up care are best managed in consultation with a healthcare provider.”
TouchBio’s Chlamydia & Gonorrhoea Rapid Test (For Female) received TGA approval in November 2024 and it will be available in the new year at retail pharmacies across the county.
To find out more information about the Chlamydia & Gonorrhoea Rapid Test, visit the website here: touchaustralia.com.au.
Like what you’re reading? Support The New England Times by making a small contribution today and help us keep delivering local news paywall-free. Support now